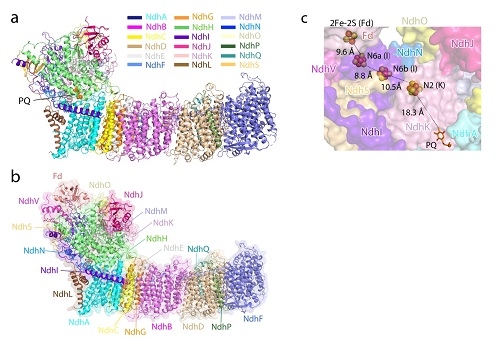
Advances in structural basic research of cyanobacteria photosynthetic ring electron transfer
Kayak Additional Accessories,Self Draining Scupper Plugs,Brackets For Paddles,Accessories For Kayak Yongsheng Outdoor Products manufactory , https://www.noahwaterkayak.com